 |  |
Synthesis Support | |
 |  |
Synthesis Support | |
|
This work was done in Prof. Jordan's group in tight collaboration with a synthetic group led by Prof. Kay Brummond. Frequently my calculations lead Prof. Brummond's group to do more experiments, and their experimental results lead me to do more calculations. Prof. Brummond's group specializes in developing and using Rh-catalyzed reactions for organic synthesis. Transition-metal catalyzed reactions typically have many possible mechanisms. Modeling such reactions can help understand their mechanisms and make improvements. I modeled Rh- and Mo-catalyzed reactions performed by Prof. Brummond's group at the B3LYP level of theory using the Gaussian 98 and Gaussian 03 packages. 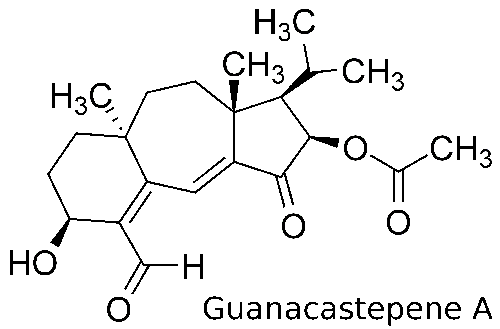 One reaction I modeled was related to an important synthetic step in the synthesis of guanacastepene A,1 a substance isolated from the fungal extracts found on the branches of the Daphnopsis Americana tree. Analogs of guanacastepene A may find use as treatments of the diseases caused by methacillin- and vancomycin-resistant pathogens. 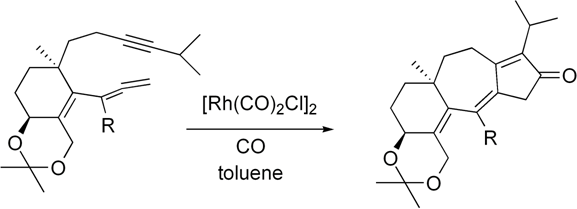 The unique feature of this step is the formation of the 7-5 ring system. If catalysts based on other transition-metal complexes are used, for example Mo(CO)6, a 6-6 ring system forms instead. Through modeling I tried to understand why the reaction proceeds the way it does when the rhodium-based catalyst is used. The answer turned out to be in the unique geometry at the rhodium center, which prevents the formation of the thermodynamically favored 6-6 ring system. 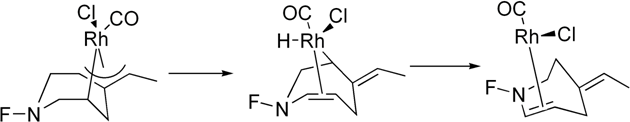 The other cycloisomerization reaction2 I modeled involved an unexpected transfer of a hydrogen atom. My modeling helped the experimentalists understand that this transfer occurs after the ring formation and how steric effects favor the transfer of this hydrogen atom as opposed to the one on the other side of the ring.
I also modeled the related reactions, in which the heteroatom and an electron withdrawing group were replaced by a carbon atom with two electron-withdrawing groups on it. 3
1 Brummond, K. M.; Gao, D. Org. Lett. 2003, 5, 3491 2 Brummond, K. M.; Chen, H.; Mitasev, B.; Casarez, A. D. Org. Lett. 2004, 6, 2161. |